
As molecularly targeted therapy continues to advance in lung cancer, racial differences in specific genetic/genomic alterations can have an important impact in the choices of therapeutics and in our understanding of the drug sensitivity/resistance profile. The most relevant genes in lung cancer described in this review include the following: EGFR, KRAS, MET, LKB1, BRAF, PIK3CA, ALK, RET, and ROS1. Commonly identified genetic/genomic alterations such as missense or nonsense mutations, small insertions or deletions, alternative splicing, and chromosomal fusion rearrangements were discussed. Relevance in current targeted therapeutic drugs was mentioned when appropriate. We also highlighted various targeted therapeutics that are currently under clinical development, such as the MET inhibitors and antibodies.
Copy number alterations in lung cancer have been studied using dense single-nucleotide polymorphism (SNP) arrays, providing us with further insight about the molecular basis of the disease. Weir et al. identified 57 significantly recurrent events from a cohort of 371 tumors. The most commonly identified event was chromosome 14q13.3 amplification, accounting for 12% of all the tumor samples. A novel proto-oncogene involved in a significant fraction of lung adenocarcinomas was identified as NKX2-1 (NK2 homeobox 1, also called TITF1), which resides in the 14q13.3 amplification interval and encodes a lineage-specific transcription factor. Interestingly, a recent study examining the TITF1 protein and genomic expression in non–small cell lung cancer (NSCLC) using integrative immunohistochemistry (for protein expression), FISH, and qPCR (for gene copy number) analysis revealed that the protein versus genomic patterns of TITF1 have opposing roles in NSCLC prognosis and may occur preferentially in different subsets of NSCLC patients with distinct oncogenic mutations. Broet et al. reported significantly higher rates of copy number gain on 16p13.13 and 16p13.11 in East Asian patients’ tumor samples while higher rates of genomic loss on 19p13.3 and 19p13.11 occurred in white patients. A novel oncogene FUS was found to be frequently associated with gain in copy number in the 16p region in lung adenocarcinoma in never smokers in addition to the finding of MYC gene copy number gain. Both EGFR and KRAS gene copy number gains have been found to occur more frequently in tumors harboring the activating mutations of the respective oncogene.
Mutations
he unprecedented advances in lung cancer genome analysis in recent years have revolutionized our understanding of the disease at a deeper molecular scale. First, the analysis of entire gene families (e.g., protein kinome, lipid kinome, and tyrosine phosphatome) as part of DNA mutational profiling of cancer genes in lung cancer unveiled vital information about the molecular structure of the disease. Protein mutations of the RAS/RAF/MEK/MAP kinase signaling pathway were studied in the first of its kind large-scale system.The study showed that serine/kinase BRAF was frequently mutated in human cancer at a frequency of 66% in malignant melanoma and at a less dramatic rate in other types of cancer including lung cancer (2% in primary adenocarcinoma). The discovery of cancer-associated mutations was driven by systemic resequencing of the cancer genome. A recent study intending to discover new somatic mutations in 188 human lung adenocarcinomas revealed over 1,000 somatic mutations after DNA sequencing of 623 genes with known or suspected cancerous activity. It identified 26 genes with a significantly high mutagenesis rate, possibly implicating them in tumorigenesis. Other frequently mutated genes include tyrosine kinases such as EGFR homolog ERBB4 and multiple Ephrin receptor genes such as EPHA3, VEGFR2 (KDR), and NTKR. These studies provide us with insight into key signaling pathways in lung adenocarcinoma tumorigenesis, which can serve as novel molecular targets for future therapeutic development.
In the following, we will provide a review with emphasis on the molecular genetic variations in several key molecular targets that are documented in lung cancer literature (EGFR, BRAF, KRAS, MET, LKB1, and PIK3CA). The review was focused also on the racial differences of these cancer genes among human populations and the implication of such differences in the future of personalized cancer therapy.
EGFR is the key paradigm of molecular targeted therapy in lung cancer, which is now commonly used in the clinical setting worldwide . Current available drugs that target EGFR can be divided into 2 categories: small-molecule EGFR tyrosine kinase inhibitor (TKI)—gefitinib and erlotinib—and monoclonal anti-EGFR antibody—cetuximab
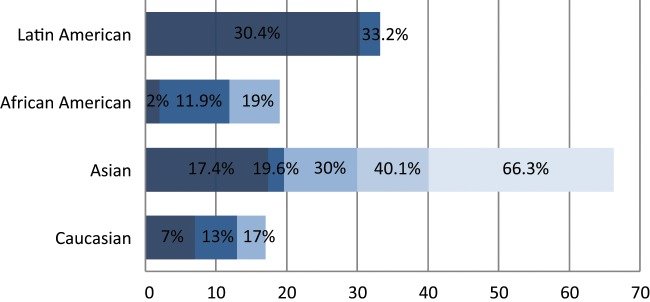
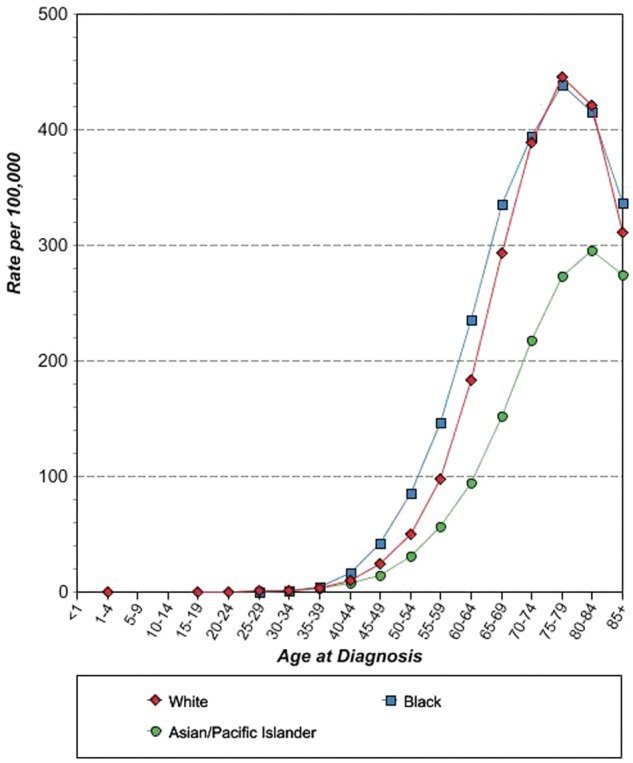
Spectrum of EGFR oncogenic driver mutations among different racial groups with NSCLC. The different color shades represent EGFR mutational rates reported by different studies. Data on the African American and Latin American cohorts are based on a limited number of studies available.Data on the Asian and white cohorts are abundant over recent years, and several representative studies were selected for graphical representation here
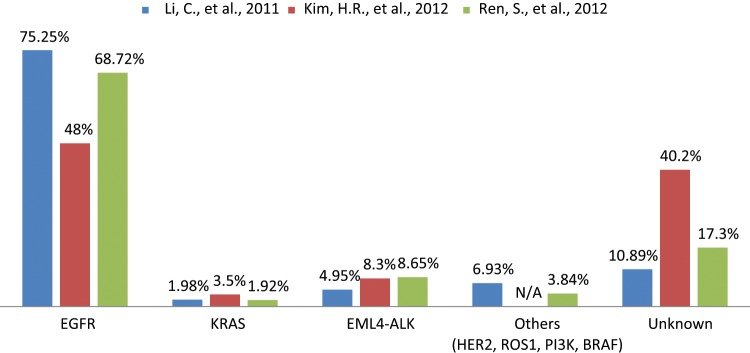
Spectrum of oncogenic driver mutations in Asian never smokers with lung adenocarcinoma.The data were collected from 3 different studies to represent the mutational frequency range of different genes among the same population. N/A = not available.
KRAS encodes a GTPase that plays the role of a central mediator of downstream growth factor receptor signaling and therefore is critical for cell proliferation, survival, and differentiation .KRAS gene mutations are uncommon in squamous cell carcinoma but can be present in approximately 15% to 25% of lung adenocarcinomas. The mutations are missense mutations primarily in codons 12 and 13 of (exon)1. In the vast majority of cases, KRAS mutations were found in EGFR wild-type tumors; hence, EGFR and KRAS mutations were mutually exclusive.Although mutant KRAS has well-established poor prognostication utility, there are conflicting data in the literature regarding its use as a predictive biomarker in NSCLC
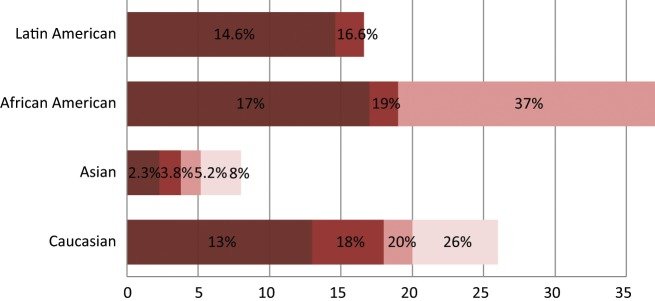
Spectrum of KRAS oncogenic driver mutations among different racial groups with NSCLC. The different color shades represent KRAS mutational rates reported by different studies. Data on the African American and Latin American cohorts are based on a limited number of available studies.Data on the white cohort are based on multiple studies including 2 meta-analyses of 22 studies with 1,470 NSCLC patients.Data on the Asian cohort are based on studies conducted in the Chinese and Korean populations.
The MET proto-oncogene is located on human chromosome 7, where EGFR and hepatocyte growth factor (HGF) genes also reside .MET encodes a receptor tyrosine kinase (RTK), which binds to its natural ligand, HGF, also called scatter factor (SF).The ligand-receptor binding induces a conformational change in the MET receptor that facilitates receptor phosphorylation and activation. In the context of malignancy, the MET-HGF/SF pathway has been strongly implicated as a mediator of pleiotropic effects such as tumor growth, survival, branching morphogenesis, motility and migration, cell scattering, invasion, tumor angiogenesis, and metastasis
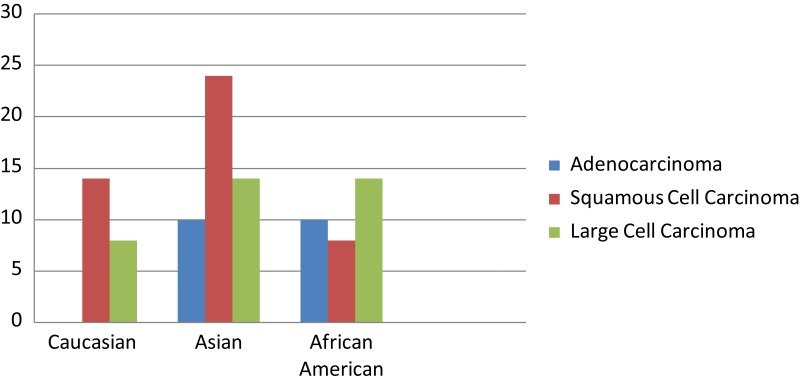
Spectrum of MET mutations among different racial groups with NSCLC. The frequency of METmutations is presented in accordance of the findings with the histological subtypes of lung cancer and racial groups.
LKB1 gene (also known as STK11) is a tumor suppressor gene located on chromosome 19p13.3 . The LKB1 gene was initially identified as the causative agent behind Peutz-Jeghers syndrome through a germline inactivating mutation.
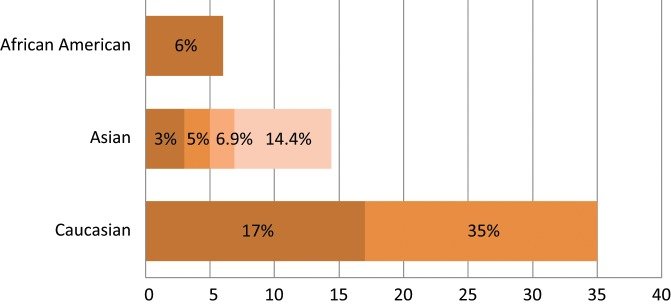
Spectrum of LKB1 oncogenic mutations among different racial groups with NSCLC..The different color shades represent LKB1 mutational rates reported by different studies.
BRAF kinase belongs to a family of serine-threonine protein kinases that includes ARAF, BRAF, and CRAF (RAF1). Mutant BRAF has been implicated in the pathogenesis of several cancers, including melanoma, NSCLC, ovarian cancer, papillary thyroid cancer, and colorectal cancer. The most commonly identified BRAF mutation is V600E, which accounts for 90% of BRAF mutations in melanoma. In NSCLC, BRAF gene mutations were identified in 1% to 3% of all samples. In a recent study that sampled 697 patients with lung adenocarcinoma, 18 patients tested positive for BRAF mutations, all of whom were white.The identified BRAF mutations were V600E (50%), G469A (39%), and D594G (11%). It is also noteworthy that no patient with a BRAF mutation had a concomitant mutation in EGFRor KRAS or a translocation in ALK. Most recently, a mutated BRAF-specific inhibitor, vemurafenib, has been approved for clinical use in V600E BRAF-mediated cutaneous melanoma. This raises the possibility of matching the BRAF inhibitor to mutated BRAFexpressing NSCLC in the future and would be worth investigating.
The PIK3CA gene encodes p110α, one of the catalytic subunits of phosphatidylinositol 3-kinases (PI3Ks), which belongs to a family of lipid kinases involved in many cellular processes, including cell growth, proliferation, differentiation, motility, and survival. PI3K is a heterodimer composed of 2 subunits: an 85-kDa regulatory subunit (p85) and a 110-kDa catalytic subunit. PI3K converts PI(4,5)P2 to PI(3,4,5)P3 on the inner leaflet of the cell membrane. PI(3,4,5)P3 recruits important downstream signaling proteins, such as AKT, to the cell membrane, resulting in increased activity of these proteins. PIK3CA was found to be mutated in over 30% of colorectal cancers.Somatic mutations in PIK3CA have been also found in 1% to 3% of all NSCLCs. Most of the mutations tended to cluster within 2 mutational hot spots. They also tended to occur more commonly in squamous cell carcinoma.PIK3CA shows significant potential as a candidate in cancer-targeted drug therapy. Currently, there are several ongoing clinical trials using PI3K inhibitors. Of interest, the PI3K inhibitor may also have a role to overcome acquired EGFR TKI–resistant disease since PIK3CA mutations have been identified in these tumor tissues in a rebiopsy study.
ALK is another tyrosine kinase receptor that is abnormal in various types of malignancies While the role of ALK in human cancer has long been recognized in NPM-ALK fusion in non-Hodgkin lymphoma,EML4-ALK fusion was documented in the literature for the first time in NSCLC only recently by Soda et al.in 2007 as a novel potential oncogenic driver mutant kinase.Approximately 3% to 7% of lung tumors harbor ALK fusions.Multiple different EML4-ALK fusion variants have been described in NSCLC, typically with varying fusion sites at EML4 but with a constant fusion site within ALK. EML4-ALK fusions are usually found in light (<10 pack years) or never smokers who tend to be of a younger age.The EML4-ALK oncogenic rearrangement was also found to be different across different racial groups. In the Asian cohort, several studies determined the incidence of the oncogenic translocation to be in the range of 2.3% to 6.7% with no significant difference compared to Asian never smokers.On the other hand, the rate of the EML4-ALK rearrangement was found to be much lower in whites, with most studies supporting a range between 1% to 3%.Interestingly, according to one study conducted on a cohort of NSCLC specimens collected from Italy and Spain, the incidence rate was found to be more similar to the Asian cohort at 7.5%.
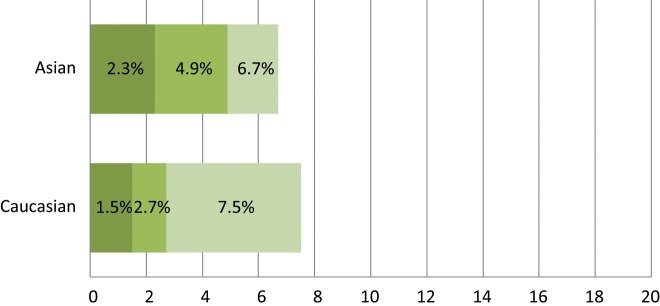
Spectrum of EML4-ALK oncogenic driver fusions among different racial groups with NSCLC.The different color shades represent EML4-ALK rates reported by different studies. Data among human populations other than white and Asian are lacking thus far.
Copyright © 2025 https://probgenbiotech.com/ All Rights Reserved.